
StudyFinder
A Prospective, Multi-center, Randomized Controlled Blinded Trial Demonstrating the Safety and Effectiveness of VNS Therapy® System as Adjunctive Therapy Versus a No Stimulation Control in Subjects With Treatment-Resistant Depression
Status: Recruiting
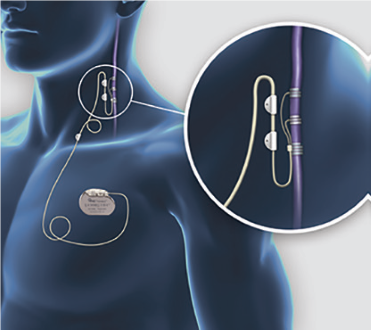
The purpose of this study is to determine whether active Vagal Nerve Stimulation (VNS) Therapy is better than no stimulation VNS Therapy in improving health outcomes for subjects with Treatment-Resistant Depression (TRD). All participants in this study will receive a VNS Therapy surgical implant, which works to reduce the symptoms of depression by sending mild electrical pulses to the vagus nerve in the neck. The vagus nerve is connected to areas of the brain associated with controlling the mood. Data will be collected on responses to study treatments, quality of life, productivity, and use of healthcare services.
Sex: Male or Female
Age Group: 18 years and over
Inclusion Criteria:
• current diagnosis of major depression for at least two years or at least 4 episodes of major depression
• have an inadequate improvement in symptoms with at least 4 antidepressant treatments
• on at least one antidepressant with a stable drug schedule for at least 4 weeks
• enrolled in Medicare or Medicare Advantage
Exclusion Criteria:
• Currently uses, or is expected to use during the study, short-wave diathermy, microwave diathermy, or therapeutic ultrasound diathermy
• acute suicide risk or suicide attempt within 6 months
• history of other major mental health diagnosis (staff will review)
• treatment with another device or experimental drug
Interventions:
Device: Vagus Nerve Stimulation (VNS)
Conditions:
Mental Health & Addiction
Keywords:
Clinics and Surgery Center (CSC), Depression, Major depression
Study Contact: Interventional Psychiatry Lab Study - ipl@umn.edu
Principal Investigator: Ziad Nahas
Phase: NA
IRB Number: SITE00000818
See this study on ClinicalTrials.gov